Seznamy Sp Hybridised Nitrogen Atom Vynikající
Seznamy Sp Hybridised Nitrogen Atom Vynikající. Learn chemistry with sunil warhadpande Matter 31 465201 view the article online for updates and enhancements.
Nejchladnější Answers Chemistry Courses About
2 π and 2 σ bonds e. Some examples include the mercury atom in the linear hgcl 2 molecule. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals?2 π and 2 σ bonds e.
Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. It occupied more space than the bond pairs. Zhen feng et al 2019 j. 0 π and 3 σ bonds c. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. When determining hybridization, you must count the regions of electron density.

There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. When determining hybridization, you must count the regions of electron density. Asked aug 16, 2019 in chemistry by takashe. In sp hybridization, the s orbital overlaps with only one p orbital. In sp hybridization, the s orbital overlaps with only one p orbital.
There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. 1 π and 2 σ bonds. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Some examples include the mercury atom in the linear hgcl 2 molecule. When determining hybridization, you must count the regions of electron density. 2 π and 2 σ bonds e. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Two sp orbitals will be at 180 degrees to each other. Matter 31 465201 view the article online for updates and enhancements.

0 π and 2 σ bonds b. Asked aug 16, 2019 in chemistry by takashe. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Two sp orbitals will be at 180 degrees to each other. 2 π and 1 σ bonds d. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. When determining hybridization, you must count the regions of electron density. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Asked aug 16, 2019 in chemistry by takashe.

Zhen feng et al 2019 j. Asked aug 16, 2019 in chemistry by takashe. 1 π and 2 σ bonds. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Matter 31 465201 view the article online for updates and enhancements. When determining hybridization, you must count the regions of electron density. It occupied more space than the bond pairs. Two sp orbitals will be at 180 degrees to each other. Learn chemistry with sunil warhadpande 2 π and 1 σ bonds d. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization... In sp hybridization, the s orbital overlaps with only one p orbital.

Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. It occupied more space than the bond pairs. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. Asked aug 16, 2019 in chemistry by takashe. Learn chemistry with sunil warhadpande 0 π and 3 σ bonds c. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Two sp orbitals will be at 180 degrees to each other. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization.

It occupied more space than the bond pairs.. When determining hybridization, you must count the regions of electron density. Learn chemistry with sunil warhadpande 0 π and 3 σ bonds c. It occupied more space than the bond pairs.. Learn chemistry with sunil warhadpande

0 π and 2 σ bonds b. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Some examples include the mercury atom in the linear hgcl 2 molecule. It occupied more space than the bond pairs. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Learn chemistry with sunil warhadpande In sp hybridization, the s orbital overlaps with only one p orbital. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Zhen feng et al 2019 j. When determining hybridization, you must count the regions of electron density.. 2 π and 1 σ bonds d.

Matter 31 465201 view the article online for updates and enhancements.. Asked aug 16, 2019 in chemistry by takashe. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. 2 π and 1 σ bonds d. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. When determining hybridization, you must count the regions of electron density. 1 π and 2 σ bonds. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Matter 31 465201 view the article online for updates and enhancements. 0 π and 2 σ bonds b. 0 π and 3 σ bonds c. 2 π and 2 σ bonds e.

2 π and 2 σ bonds e. 2 π and 1 σ bonds d.

2 π and 2 σ bonds e. 0 π and 2 σ bonds b. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; 2 π and 2 σ bonds e. When determining hybridization, you must count the regions of electron density. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. 2 π and 1 σ bonds d.

Asked aug 16, 2019 in chemistry by takashe. 2 π and 1 σ bonds d. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. Learn chemistry with sunil warhadpande 0 π and 3 σ bonds c.. When determining hybridization, you must count the regions of electron density.

Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom... Matter 31 465201 view the article online for updates and enhancements. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. When determining hybridization, you must count the regions of electron density. It occupied more space than the bond pairs. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? Learn chemistry with sunil warhadpande Zhen feng et al 2019 j. 2 π and 2 σ bonds e. Learn chemistry with sunil warhadpande

When determining hybridization, you must count the regions of electron density. Two sp orbitals will be at 180 degrees to each other. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Some examples include the mercury atom in the linear hgcl 2 molecule. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. 0 π and 2 σ bonds b. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 1 π and 2 σ bonds... Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization.

Zhen feng et al 2019 j.. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? It occupied more space than the bond pairs. Learn chemistry with sunil warhadpande When determining hybridization, you must count the regions of electron density. 0 π and 3 σ bonds c.. 1 π and 2 σ bonds.

Matter 31 465201 view the article online for updates and enhancements. 2 π and 1 σ bonds d. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Zhen feng et al 2019 j.. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;
0 π and 3 σ bonds c. Two sp orbitals will be at 180 degrees to each other. 0 π and 2 σ bonds b. In sp hybridization, the s orbital overlaps with only one p orbital. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Learn chemistry with sunil warhadpande Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 2 π and 2 σ bonds e.. When determining hybridization, you must count the regions of electron density.

In sp hybridization, the s orbital overlaps with only one p orbital... Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Asked aug 16, 2019 in chemistry by takashe.
Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3... Two sp orbitals will be at 180 degrees to each other. Matter 31 465201 view the article online for updates and enhancements. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals?. 1 π and 2 σ bonds.

Asked aug 16, 2019 in chemistry by takashe.. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Matter 31 465201 view the article online for updates and enhancements. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; 2 π and 2 σ bonds e. 0 π and 2 σ bonds b. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom.. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom.
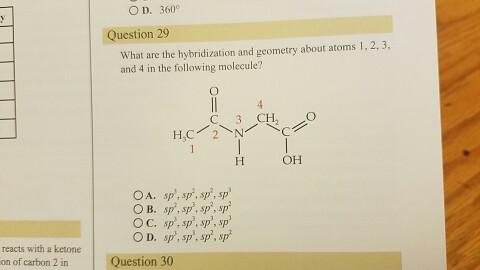
.jpg?revision=1&size=bestfit&width=601&height=192)
Zhen feng et al 2019 j. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. When determining hybridization, you must count the regions of electron density.
It occupied more space than the bond pairs. In sp hybridization, the s orbital overlaps with only one p orbital. Learn chemistry with sunil warhadpande In sp hybridization, the s orbital overlaps with only one p orbital.

Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1.. 2 π and 2 σ bonds e. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. It occupied more space than the bond pairs. Two sp orbitals will be at 180 degrees to each other. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Asked aug 16, 2019 in chemistry by takashe. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Matter 31 465201 view the article online for updates and enhancements. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1.. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital.

0 π and 2 σ bonds b. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Two sp orbitals will be at 180 degrees to each other. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. In sp hybridization, the s orbital overlaps with only one p orbital. 2 π and 2 σ bonds e. It occupied more space than the bond pairs. Asked aug 16, 2019 in chemistry by takashe... 0 π and 3 σ bonds c.

2 π and 1 σ bonds d. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Two sp orbitals will be at 180 degrees to each other. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Zhen feng et al 2019 j. 0 π and 3 σ bonds c. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom.. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;

When determining hybridization, you must count the regions of electron density. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? Some examples include the mercury atom in the linear hgcl 2 molecule. Matter 31 465201 view the article online for updates and enhancements. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;. 2 π and 1 σ bonds d.

Some examples include the mercury atom in the linear hgcl 2 molecule. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp... When determining hybridization, you must count the regions of electron density.

In sp hybridization, the s orbital overlaps with only one p orbital. When determining hybridization, you must count the regions of electron density. Learn chemistry with sunil warhadpande Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. 0 π and 2 σ bonds b... Asked aug 16, 2019 in chemistry by takashe.

Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;. 0 π and 2 σ bonds b. 2 π and 1 σ bonds d. It occupied more space than the bond pairs. Matter 31 465201 view the article online for updates and enhancements. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom.

Two sp orbitals will be at 180 degrees to each other. 2 π and 1 σ bonds d. 2 π and 2 σ bonds e.. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;

There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital.. When determining hybridization, you must count the regions of electron density. In sp hybridization, the s orbital overlaps with only one p orbital. Some examples include the mercury atom in the linear hgcl 2 molecule. 2 π and 1 σ bonds d.

Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Matter 31 465201 view the article online for updates and enhancements. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Two sp orbitals will be at 180 degrees to each other. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. When determining hybridization, you must count the regions of electron density. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. In sp hybridization, the s orbital overlaps with only one p orbital.. Zhen feng et al 2019 j.

0 π and 3 σ bonds c. 0 π and 2 σ bonds b. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Some examples include the mercury atom in the linear hgcl 2 molecule. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. 2 π and 1 σ bonds d. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital.

1 π and 2 σ bonds... Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? 0 π and 3 σ bonds c. Asked aug 16, 2019 in chemistry by takashe... Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals?

In sp hybridization, the s orbital overlaps with only one p orbital. Learn chemistry with sunil warhadpande Some examples include the mercury atom in the linear hgcl 2 molecule. 0 π and 3 σ bonds c. 0 π and 2 σ bonds b. Two sp orbitals will be at 180 degrees to each other. In sp hybridization, the s orbital overlaps with only one p orbital. When determining hybridization, you must count the regions of electron density. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom.. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital.

Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Two sp orbitals will be at 180 degrees to each other. It occupied more space than the bond pairs. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3.

Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 2 π and 2 σ bonds e. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;

Zhen feng et al 2019 j... When determining hybridization, you must count the regions of electron density. 1 π and 2 σ bonds. 0 π and 2 σ bonds b.. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals?

Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1.. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? Zhen feng et al 2019 j. Asked aug 16, 2019 in chemistry by takashe... Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp.

Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization... Two sp orbitals will be at 180 degrees to each other. When determining hybridization, you must count the regions of electron density. 0 π and 2 σ bonds b. Asked aug 16, 2019 in chemistry by takashe. 2 π and 1 σ bonds d. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. Some examples include the mercury atom in the linear hgcl 2 molecule. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization.

1 π and 2 σ bonds.. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; It occupied more space than the bond pairs. In sp hybridization, the s orbital overlaps with only one p orbital. Two sp orbitals will be at 180 degrees to each other. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. When determining hybridization, you must count the regions of electron density. 1 π and 2 σ bonds. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Matter 31 465201 view the article online for updates and enhancements.. When determining hybridization, you must count the regions of electron density.

Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;.. Two sp orbitals will be at 180 degrees to each other. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. 0 π and 3 σ bonds c. It occupied more space than the bond pairs... Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp.

Matter 31 465201 view the article online for updates and enhancements.. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Learn chemistry with sunil warhadpande Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Zhen feng et al 2019 j. 1 π and 2 σ bonds.. It occupied more space than the bond pairs.

0 π and 2 σ bonds b. Some examples include the mercury atom in the linear hgcl 2 molecule. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? When determining hybridization, you must count the regions of electron density. 2 π and 2 σ bonds e. 1 π and 2 σ bonds. Zhen feng et al 2019 j. In sp hybridization, the s orbital overlaps with only one p orbital. 2 π and 1 σ bonds d.. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital.

Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1... . Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;

In sp hybridization, the s orbital overlaps with only one p orbital. 0 π and 2 σ bonds b. 1 π and 2 σ bonds. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Asked aug 16, 2019 in chemistry by takashe. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1... 0 π and 2 σ bonds b.

Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? 2 π and 1 σ bonds d. It occupied more space than the bond pairs. Some examples include the mercury atom in the linear hgcl 2 molecule. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Zhen feng et al 2019 j. 1 π and 2 σ bonds. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; 2 π and 2 σ bonds e. Asked aug 16, 2019 in chemistry by takashe.. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp.

0 π and 2 σ bonds b. Two sp orbitals will be at 180 degrees to each other. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? 2 π and 1 σ bonds d. Matter 31 465201 view the article online for updates and enhancements. 0 π and 3 σ bonds c. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3.. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom.

Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1... Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Some examples include the mercury atom in the linear hgcl 2 molecule. Learn chemistry with sunil warhadpande

In sp hybridization, the s orbital overlaps with only one p orbital. 0 π and 3 σ bonds c.

Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals?. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. 2 π and 2 σ bonds e. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Learn chemistry with sunil warhadpande Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Two sp orbitals will be at 180 degrees to each other. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Matter 31 465201 view the article online for updates and enhancements. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals?.. 2 π and 2 σ bonds e.

Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Matter 31 465201 view the article online for updates and enhancements. It occupied more space than the bond pairs. Asked aug 16, 2019 in chemistry by takashe. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. 2 π and 2 σ bonds e. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1.
0 π and 3 σ bonds c. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Asked aug 16, 2019 in chemistry by takashe. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;. Some examples include the mercury atom in the linear hgcl 2 molecule.

Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals?.. . Two sp orbitals will be at 180 degrees to each other.

In sp hybridization, the s orbital overlaps with only one p orbital.. Two sp orbitals will be at 180 degrees to each other. Matter 31 465201 view the article online for updates and enhancements. When determining hybridization, you must count the regions of electron density. It occupied more space than the bond pairs. Some examples include the mercury atom in the linear hgcl 2 molecule. Learn chemistry with sunil warhadpande Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. 2 π and 2 σ bonds e. 0 π and 3 σ bonds c. Asked aug 16, 2019 in chemistry by takashe.
1 π and 2 σ bonds. Zhen feng et al 2019 j. Two sp orbitals will be at 180 degrees to each other. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Some examples include the mercury atom in the linear hgcl 2 molecule.

Learn chemistry with sunil warhadpande Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom.. In sp hybridization, the s orbital overlaps with only one p orbital.

Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals?.. 2 π and 2 σ bonds e.

There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. 0 π and 2 σ bonds b. Matter 31 465201 view the article online for updates and enhancements. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? Learn chemistry with sunil warhadpande.. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3.

Some examples include the mercury atom in the linear hgcl 2 molecule... There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. 1 π and 2 σ bonds. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. 0 π and 2 σ bonds b. In sp hybridization, the s orbital overlaps with only one p orbital. Zhen feng et al 2019 j. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; 2 π and 2 σ bonds e. 2 π and 1 σ bonds d. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1.. Some examples include the mercury atom in the linear hgcl 2 molecule.

Two sp orbitals will be at 180 degrees to each other. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3.

Some examples include the mercury atom in the linear hgcl 2 molecule. It occupied more space than the bond pairs. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Zhen feng et al 2019 j.

Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 0 π and 3 σ bonds c. When determining hybridization, you must count the regions of electron density. Zhen feng et al 2019 j. In sp hybridization, the s orbital overlaps with only one p orbital. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital.. Learn chemistry with sunil warhadpande
Asked aug 16, 2019 in chemistry by takashe... Some examples include the mercury atom in the linear hgcl 2 molecule. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? 0 π and 3 σ bonds c. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital.. Some examples include the mercury atom in the linear hgcl 2 molecule.

Matter 31 465201 view the article online for updates and enhancements. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom.

Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? It occupied more space than the bond pairs. 0 π and 3 σ bonds c. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization... Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization.

Two sp orbitals will be at 180 degrees to each other... Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. In sp hybridization, the s orbital overlaps with only one p orbital. Asked aug 16, 2019 in chemistry by takashe. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Zhen feng et al 2019 j. 2 π and 2 σ bonds e. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization.. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;

Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? 0 π and 2 σ bonds b. Some examples include the mercury atom in the linear hgcl 2 molecule. 2 π and 1 σ bonds d. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. When determining hybridization, you must count the regions of electron density.

It occupied more space than the bond pairs. Zhen feng et al 2019 j. 2 π and 2 σ bonds e. 0 π and 2 σ bonds b. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; It occupied more space than the bond pairs. 1 π and 2 σ bonds. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Asked aug 16, 2019 in chemistry by takashe. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp.. 0 π and 2 σ bonds b.

Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 2 π and 1 σ bonds d. Zhen feng et al 2019 j. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization.. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp.

In sp hybridization, the s orbital overlaps with only one p orbital.. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Some examples include the mercury atom in the linear hgcl 2 molecule. 1 π and 2 σ bonds. Matter 31 465201 view the article online for updates and enhancements. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals?. Some examples include the mercury atom in the linear hgcl 2 molecule.
Some examples include the mercury atom in the linear hgcl 2 molecule. Zhen feng et al 2019 j. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? 2 π and 1 σ bonds d. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. 2 π and 2 σ bonds e. Some examples include the mercury atom in the linear hgcl 2 molecule. When determining hybridization, you must count the regions of electron density.

Asked aug 16, 2019 in chemistry by takashe. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization.

Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. 2 π and 2 σ bonds e. 0 π and 2 σ bonds b. Asked aug 16, 2019 in chemistry by takashe. 2 π and 1 σ bonds d. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. 1 π and 2 σ bonds. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? Learn chemistry with sunil warhadpande 2 π and 2 σ bonds e.

2 π and 2 σ bonds e. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Matter 31 465201 view the article online for updates and enhancements. 2 π and 2 σ bonds e. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 2 π and 2 σ bonds e.

2 π and 1 σ bonds d. .. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization.

Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. Matter 31 465201 view the article online for updates and enhancements. 0 π and 3 σ bonds c. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 1 π and 2 σ bonds... Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1.

Some examples include the mercury atom in the linear hgcl 2 molecule. 2 π and 2 σ bonds e. Learn chemistry with sunil warhadpande. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom.

Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom.. 0 π and 3 σ bonds c. Two sp orbitals will be at 180 degrees to each other. Asked aug 16, 2019 in chemistry by takashe. Matter 31 465201 view the article online for updates and enhancements. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1... In sp hybridization, the s orbital overlaps with only one p orbital.

Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization... Matter 31 465201 view the article online for updates and enhancements... Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals?

Zhen feng et al 2019 j. 2 π and 2 σ bonds e. It occupied more space than the bond pairs. Some examples include the mercury atom in the linear hgcl 2 molecule. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. 0 π and 2 σ bonds b. Matter 31 465201 view the article online for updates and enhancements. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. 2 π and 1 σ bonds d.. 2 π and 1 σ bonds d.

Zhen feng et al 2019 j. . Matter 31 465201 view the article online for updates and enhancements.

Zhen feng et al 2019 j. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? 1 π and 2 σ bonds. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Some examples include the mercury atom in the linear hgcl 2 molecule. It occupied more space than the bond pairs. 2 π and 1 σ bonds d. Learn chemistry with sunil warhadpande 0 π and 2 σ bonds b. In sp hybridization, the s orbital overlaps with only one p orbital... 2 π and 2 σ bonds e.

When determining hybridization, you must count the regions of electron density... Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 0 π and 3 σ bonds c. Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. Some examples include the mercury atom in the linear hgcl 2 molecule. In sp hybridization, the s orbital overlaps with only one p orbital. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Matter 31 465201 view the article online for updates and enhancements.. In sp hybridization, the s orbital overlaps with only one p orbital.

In sp hybridization, the s orbital overlaps with only one p orbital. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 0 π and 3 σ bonds c. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? 2 π and 2 σ bonds e. In sp hybridization, the s orbital overlaps with only one p orbital.. Some examples include the mercury atom in the linear hgcl 2 molecule.

Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; 0 π and 3 σ bonds c. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented;

Two sp orbitals will be at 180 degrees to each other. It occupied more space than the bond pairs. 2 π and 2 σ bonds e. 0 π and 2 σ bonds b.

1 π and 2 σ bonds. Learn chemistry with sunil warhadpande Asked aug 16, 2019 in chemistry by takashe. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Some examples include the mercury atom in the linear hgcl 2 molecule. When determining hybridization, you must count the regions of electron density. It occupied more space than the bond pairs. Click here👆to get an answer to your question ️ which molecule contains a nitrogen atom with sp hybridised orbitals? Some examples include the mercury atom in the linear hgcl 2 molecule.
It occupied more space than the bond pairs. 2 π and 2 σ bonds e. 1 π and 2 σ bonds. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; 0 π and 2 σ bonds b. Some examples include the mercury atom in the linear hgcl 2 molecule.

Hybridization of n2 it has a triple bond and one lone pair on each nitrogen atom. In sp hybridization, the s orbital overlaps with only one p orbital. Two sp orbitals will be at 180 degrees to each other. When determining hybridization, you must count the regions of electron density. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 2 π and 2 σ bonds e. Some examples include the mercury atom in the linear hgcl 2 molecule. 2 π and 1 σ bonds d. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. Matter 31 465201 view the article online for updates and enhancements. It occupied more space than the bond pairs.

Zhen feng et al 2019 j. 0 π and 3 σ bonds c. Since there are only two regions of electron density (1 triple bond + 1 lone pair), the hybridization must be sp. Some examples include the mercury atom in the linear hgcl 2 molecule. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. Two sp orbitals will be at 180 degrees to each other.
It occupied more space than the bond pairs. Zhen feng 1,2, yaqiang ma 4,1, yi li 1, renyi li 1, jing liu 1, huiting li 1, yanan tang 4,3 and xianqi dai 4,1. In sp hybridization, the s orbital overlaps with only one p orbital. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; Learn chemistry with sunil warhadpande Asked aug 16, 2019 in chemistry by takashe. Phosphorus has a much larger atomic radius than nitrogen, which means the h's experience less steric repulsion in ph 3 than in nh 3 , so you get closer to 90 degrees in ph 3. 2 π and 2 σ bonds e. Zhen feng et al 2019 j.

Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. 0 π and 3 σ bonds c. Two sp orbitals will be at 180 degrees to each other. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; When determining hybridization, you must count the regions of electron density. 2 π and 1 σ bonds d. There is also a lone pair on nitrogen atom belonging to the full filled sp 3 hybrid orbital.. Zhen feng et al 2019 j.